Water is weird…and I point this out in an admiring manner… Water by itself is weird…it is a “polar molecule”…think “tiny magnets”….but when it changes phase from a liquid to a solid or from a liquid to a vapor the weirdness goes to levels that mere mortals are left shaking their heads….or it leads them to drinking….apparently wine. More about the wine drinking later.
Freeze-thaw damage, spalling and efflorescence are related in an amazing strange way. In freeze-thaw damage water changes from a liquid to a solid (Photograph 1). In spalling (Photograph 2) and efflorescence (Photograph 3) water changes from a liquid to a vapor.

Photograph 1: Freeze-Thaw Damage – Water changes from a liquid to a solid.

Photograph 2: Spalling – Water changes from a liquid to a vapor.

Photograph 3: Efflorescence – Water changes from a liquid to a vapor.
Freeze-thaw damage does not occur because when water freezes its volume increases by 9 percent. Note that the water does increase in volume, but this is not what leads to the freeze-thaw issues. Let me repeat, this change in volume is not what leads to freeze-thaw issues.1
The planet is covered by oceans. The oceans are filled with salt. When salt water freezes the salt is left behind. That is why icebergs (Photograph 4) do not have salt in them.2 The salt concentration of the surrounding ocean goes up when the salt water freezes.

Photograph 4: Icebergs - When salt water freezes the salt is left behind that is why ice-bergs do not have salt in them.
When water changes phase from a liquid to a solid… stuff is left behind. Let me repeat that…because it is a big deal and folks don’t get it…when water changes phase from a liquid to a solid…stuff is left behind.
If you have soluble salts in brick or concrete and they are in solution because the brick or concrete is wet because of bad water management. When the water changes phase by freezing the concentration of salt goes up. The Second Law of Thermodynamics does not like that and water from elsewhere in the brick or concrete rushes to the increased concentration of salt to dilute the concentration. The forces that are involved can be huge…thousands of PSI….osmotic forces…and the brick and concrete burst apart. When the phase change is from liquid to solid and there is damage we call it freeze-thaw.
The osmosis pressures can exceed the cohesive strength of most porous materials and they literally blow apart.
Diffusion Vapor Pressure 0.3 to 0.5 psi (2.1 to 3.5 kPa)
Capillary Pressure 300 to 500 psi (2.1 to 3.5 MPa)
Osmotic Pressure 3,000 to 5,000 psi (21 to 35 MPa)
In concrete we address the issue by adding voids…airspaces…in the concrete….air entrained concrete to reduce the tremendous hydrostatic pressures to allow the water to squirt into the tiny air bubbles.3
In brick we address the issue by manipulating the voids in the brick by controlling the brick kiln temperature or by changing the chemistry of the mortar to attract the dissolved salt water….sacrificial mortar. To determine the effect of voids within brick you can measure the brick to determine the “critical degree of saturation”4 (yes, we were here before….”BSI-047: Thick as a Brick”, May 2010). Usually, the building already tells you if the brick is good or bad if it is old and if you look…no damage no problem. There is a catch, however, there always is. Note that the “no damage” part could be because things don’t get wet enough to matter…or the brick is good….or both. Further note that you can make things work with bad brick by making sure the brick does not get really wet….duh!!!
Guess what? Here it comes. When water changes phase from a liquid to a vapor, stuff is also left behind. Woah.…. Let me repeat that…because it is a big deal and folks don’t get it…when water changes phase from a liquid to a vapor…stuff is left behind. If it is salt…bad things happen. Sound familiar? We call it spalling and efflorescence (yes, we were here way back when…"BSI-011: Capillarity—Small Sacrifices", July 2007).
Figure 1 shows water with salt in solution traveling through a porous material by capillary flow to a surface where evaporation occurs. As the capillary water evaporates from the surface the salt is left behind. As water continues to evaporate from the surface this leads to an ever-increasing concentration of salt in the material. This increasing concentration of salt disturbs matters and to re-establish equilibrium water rushes towards the salt to dilute the concentration of salt. This can lead to huge hydrostatic pressures within the porous material caused by “osmosis”.
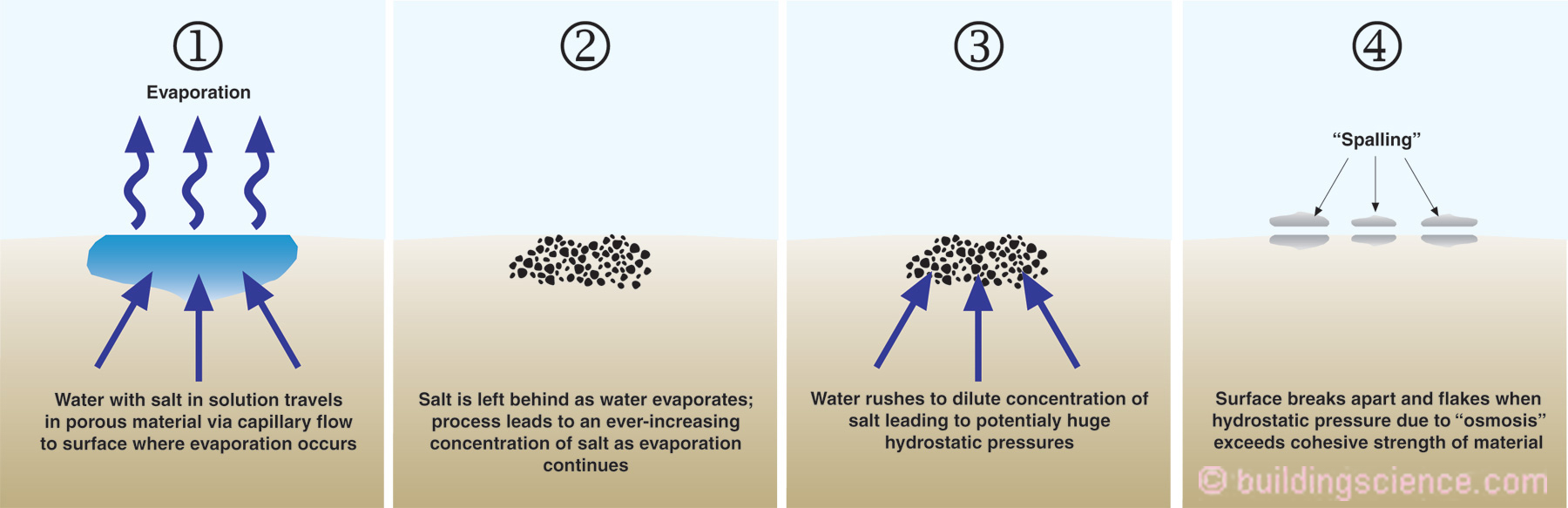
Figure 1: Spalling of Porous Materials – Without salt present evaporation of water from porous surfaces can often be easily managed – with salt present significant damage can occur.
Photograph 3 shows salt on the above grade exposed surface of a brick foundation. Water carries salt in solution to the surface of the brick where the water evaporates leaving behind the salt on the surface of the brick in the form of salt crystals. The white deposits of mineral salts are called “efflorescence”. When the crystallization of salts happens beneath the surface of the brick-mortar matrix the phenomenon is called “sub surface efflorescence” or “subflorescence”. If the subflorescence leads to sufficiently strong osmotic pressures – greater than the strength of the brick - and the brick flakes apart the damage is called “spalling”.
Here is where the drinking part comes in – “ice wines” and “vin santo” (Photograph 5). One relies on a change in phase from a liquid to a solid and the other relies on a change in phase from a liquid to a vapor. Ice-wines are incredibly tasty because if you leave the grapes on the vine and the grapes freeze, stuff is left behind. Sound familiar? When you crush the grapes and get rid of the frozen water what is left behind are the sugars and the dissolved solids. The flavor is enhanced. The Germans figured this out first because they have winter in Germany. Germans, thank you for “ice wine”.

Photograph 5: Ice Wine and Vin Santo – Nothing else needs to be said beyond good bye liquid water.
In Italy they do not have much of a winter but they have a lot of sun (Photograph 6). If they leave the grapes in the sun the water in them evaporates and leaves behind the sugars and the dissolved solids. Sound familiar? We have “vin santo”. Italians…Friends, Romans, Countrymen….thank you for “vin santo”.

Photograph 6: Happy Italian Sommelier – I took this photo just outside of Sienna, Italy. Learned about phase change from an expert.
The French gave us “sauternes”….that also involves getting rid of liquid water…but how that is done is for another time. We will need a chemical engineer/microbiologist…or just a Nobel Prize winner in physics5 to do that story justice.
You want to have some fun…put a German, an Italian and a Frenchman in a room together with an “ice wine”, a “vin santo” and a “sauterne”…Invite me if you can pull this off. Oh, also make it “blind” taste testing and get a lawyer that knows wine to officiate….maybe a guy named Parker….
Footnotes
1. This has been known for more than a half a century. See this link and marvel at the genius of G.G. Litvan. Litvan presented his ideas well before 1976….I seem to recall when I, as a youngster, sat at the feet of the “legends” of building science, Litvan first presented his ideas around the time my Toronto Maple Leafs won their last Stanley Cup: https://nrc-publications.canada.ca/eng/view/ft/?id=5817f9d2-15a6-426c-83ee-fe804caef59e. Experiments were subsequently done with liquids that did not change in volume when they went from a liquid to a solid. No salt, no damage. When salt was added damage occurred. So…. you can get freeze-thaw damage when there is no change in volume but when there is a change in salt concentration….
2. We call this the Linda Ronstadt effect. Allegedly, when she was dating Jerry Brown when he was the Governor of California she pointed this out to him and that is why he wanted to tow icebergs from Alaska to California to solve California's water problem. Note the word “we”….apparently “we” were wrong…even though “we” were saying this for years. Some youngsters “fact checked” this “story”…ah graduate students… It was not Jerry Brown but the city of Ventura (see: https://www.latimes.com/archives/la-xpm-1990-06-06-me-455-story.html). Now it is possible that Linda Ronstadt might have been consulted by Ventura city officials, however this has not been confirmed…nor denied….
3. We call this the Don Ho effect. Don Ho was a Hawaiian lounge singer who had a hit record in 1966 called “Tiny Bubbles”. We used to make our own air entrained concrete when I was first starting by adding a surfactant. Our favorite surfactant was liquid “Ivory Snow”. Yup, we made “concrete hootch”…”concrete home-brew”. There is more to this story but some stories I can’t tell even in a footnote….ask me if we ever meet.
4. The two key papers on the subject are: Ritchie, T. “Factors Affecting Frost Damage to Clay Bricks.” Building Research Note No. 62, NRCC, 1968; and Fagerlund, G. “The critical degree of saturation method of assessing the freeze/thaw resistance of concrete.” Materials and Structures 10:58, 1977.
5. A poet once said, 'The whole universe is in a glass of wine.' We will probably never know in what sense he meant it, for poets do not write to be understood. But it is true that if we look at a glass of wine closely enough, we see the entire universe. There are the things of physics: the twisting liquid which evaporates depending on the wind and weather, the reflection in the glass; and our imagination adds atoms. The glass is a distillation of the earth's rocks, and in its composition, we see the secrets of the universe's age, and the evolution of stars. What strange array of chemicals are in the wine? How did they come to be? There are the ferments, the enzymes, the substrates, and the products. There in wine is found the great generalization; all life is fermentation. Nobody can discover the chemistry of wine without discovering, as did Louis Pasteur, the cause of much disease. How vivid is the claret, pressing its existence into the consciousness that watches it! If our small minds, for some convenience, divide this glass of wine, this universe, into parts -- physics, biology, geology, astronomy, psychology, and so on -- remember that nature does not know it! So let us put it all back together, not forgetting ultimately what it is for. Let it give us one more final pleasure; drink it and forget it all!”
― Richard P. Feynman, The Feynman Lectures on Physics, Volume I, Lecture 3, Section 3-7, Paragraph 51963
